The
BPIA annual meeting brings together regulators to raise and resolve issues and comment on emerging policies and regulations. The event focused on three themes:
1. Global Regulatory: Anything New with Global Regulation of Biological Products?
Representatives from both the
US Environmental Protection Agency (EPA) and the
Canada Pest Management Regulatory Agency (PMRA) provided a regulatory overview for the US and Canada, including. the status of updated guidance for the registration of microbial and non-conventional pest control products in Canada:
- Registration requirements for biopesticides used on cannabis and industrial hemp grown for flower must include an evaluation of potential consumer exposure.
- In Canada, the data requirements for dsRNA-based pesticides are determined case-by-case. A pre-submission consultation is required.
A framework for data requirements for microorganisms in the EU was discussed. Current data requirements Regulation (EU) 2022/1439 has been updated to Regulation (EU) 2022/1440. The transition period is up to May 2023, i.e., applicants can choose to submit a dossier following the old or the new data requirements. After May 2023, it is compulsory to follow the new data requirements.
An updated crosswalks file is available here.
2. Bioherbicides: Challenges and Opportunities
A speaker panel from industry discussed challenges and opportunities in developing bioherbicides, grouped as:
- Microbial herbicides include viruses, bacteria, fungi, and oomycetes – live or killed (including fermentation products)
- Biochemical herbicides include microbial extracts, botanical extracts – allelochemicals, essential oils, and seed oils – and organic acids, fatty acid salts.
Over 30 microbial herbicides have been identified worldwide, but fewer than a third are commercially available due to challenges with narrow spectrum of activity, temperature and moisture requirements of live microbes, and the high cost of fermentation, downstream processing, and formulation.Most biological products companies face similar technical and regulatory (cost and timing of federal registration) challenges.
3. Plant Growth Regulators:
Where is the Market for Plant Growth Regulators (PGRs) Going in the Face of Evolving Regulations in the US and EU?
Presenters provided 2021 general statistics, below, for the global biocontrol market and the role of PGRs along with an overview of the
EPA’s draft guidance for Plant Regulator Products and Claims, including Plant Biostimulants.
PGRs represent 19% of the global biocontrol market:
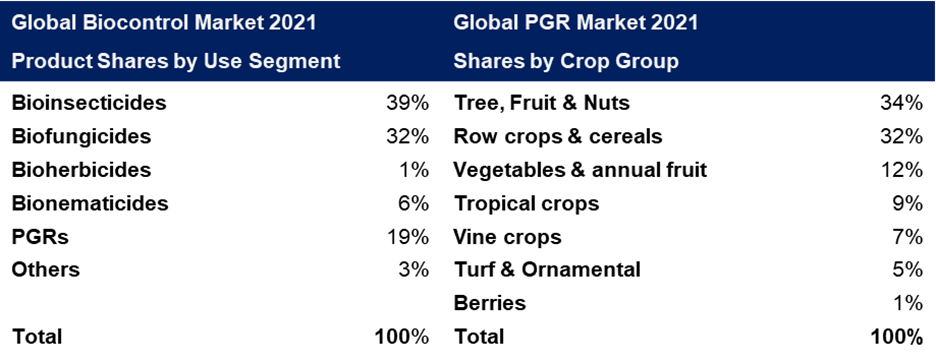 Table adapted from DunhamTrimmer® Global Biocontrol Market 2021
Table adapted from DunhamTrimmer® Global Biocontrol Market 2021
The guidance is still in draft but is being used by different US States as a reference for determining whether a biostimulant is really a PGR. Products with claims considered as plant regulator are regulated as pesticides under
the Federal Insecticide, Fungicide, and Rodenticide Act (FIFRA). EPA will not have jurisdiction over any products not considered PGRs. Some products being sold as plant biostimulants may trigger regulation under FIFRA as plant regulators, based on how they function. Some active ingredients have dual use and must be registered as PGRs, regardless of the claims being made.
The Microbiome Movement AgBiotech Summit focuses on industry and regulatory initiatives to gain consensus on biostimulants, biopesticides, and biofungicides as the field evolves. Areas of focus included:
- Enhancing understanding of the plant-soil microbiome by characterizing microbial functionality at the molecular level, using microbes to improve plant health, and understanding how plants use soil and endophytic microbes for nutrient absorption.
- Overcoming the formulation, manufacturing, & scalability bottleneck without compromising product quality; most of the difficulties to overcome were shared across different companies, including, production scale-up, variability in microbial population, and lack of capacity at the fermentation or formulation stage.
- Challenges in delivering biological products to target application sites and understanding the different application requirements for row and specialty crops.
- Commercial relevance of biologicals.
- Implications for future development of agricultural biological products, such as converting the diversity of environment microbes into novel AgTech products and exploring possibilities of microbial functionality.
Both conferences had a feedback session from a panel of growers on their experiences using biological products. Overall, we are seeing an increase in using biological products in the United States. Wider adoption will depend on profit and how biological products fit into routine farm operations.
Even as the market need for biological products is increasing, we are not yet seeing a corresponding clear regulatory guidance from Europe. Industry and regulators are striving for regulatory consensus on biostimulants, biopesticides, and biofungicides. This could be a reflection of the nature of biological products themselves; microorganisms are highly diverse, resulting in many seemingly conditional data requirements and the need for pre-submission consultation. These conferences involve collaboration among producers, regulators, consultants, academia, etc., and demonstrate that all stakeholders are working together to advance this emerging technology in an effort to facilitate a transformation to sustainable agriculture.
Interested in learning more about the topic of biologicals? Smithers conducts both conventional and biological testing for agrochemical registrations in all regions. Contact Dr. Malekani to discuss your questions about environmental testing of biological products.
References
Guidance for the Registration of Microbial Pest Control Agents and Products https://www.canada.ca/en/healthcanada/services/consumer-product-safety/reports-publications/pesticides-pest-management/policiesguidelines/guidance-registration-microbial-pest-control-agent-products.html
OECD Working Document on Considerations for the Environmental Risk Assessment of the Application of Sprayed or Externally Applied ds-RNA-based Pesticides, Series on Pesticides No. 104 [ENV/JM/MONO (2020)26]
https://www.oecd.org/officialdocuments/publicdisplaydocumentpdf/?cote=env/jm/mono(2020)26&doclanguage=en
OECD Guidance Document for the Regulatory Framework for the Microorganism Group: Bacteriophages, Series on Pesticides No. 108 [ENV/CBC/MONO(2022)40]
https://www.oecd.org/officialdocuments/publicdisplaydocumentpdf/?cote=env/cbc/mono(2022)40&doclanguage=en\
Crosswalks EU PPP Microorganisms - active substance application (product) to New Data Requirements Regulation (EU) 2022/1439 & Regulation (EU) 2022/1440
Draft Guidance for Plant Regulator Products and Claims, Including Plant Biostimulants https://www.epa.gov/sites/default/files/2020-11/documents/pbs-guidance-updated-draft-guidance-document-2020-11-13_0.pdf
.jpg?ext=.jpg)
